Global Pompe Disease Treatment Market, Share, Demand, Growth, and Report 2024-2032
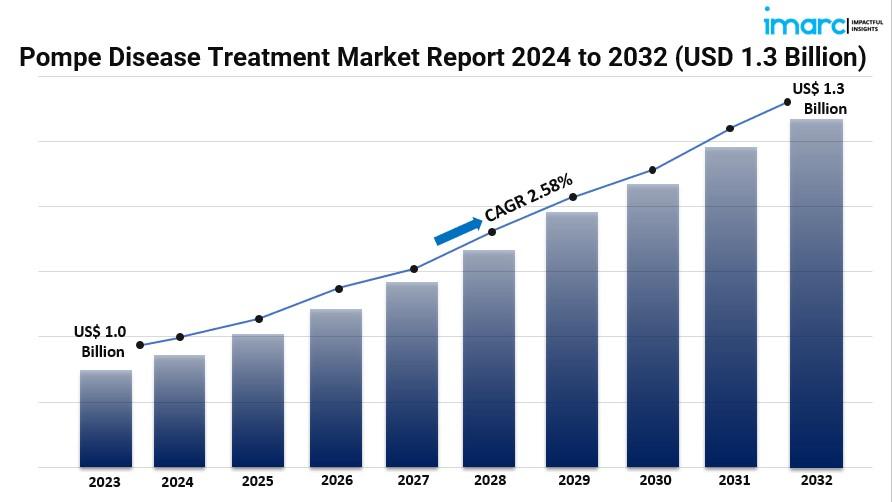
The latest report by IMARC Group, titled “Pompe Disease Treatment Market by Treatment (Enzyme Replacement Therapy (ERT), Substrate Reduction Therapy (SRT), Chaperone-Advanced Replacement Therapy (CART), and Others), Route of Administration (Oral, Intravenous, and Others), Distribution Channel (Hospital and Clinics Pharmacies, Retail Pharmacies, Online Pharmacies, and Others), Indication Type (Infantile-Onset Pompe Disease (IOPD), Classic Infantile Form, Non-Classic Infantile Form, Late-Onset Pompe Disease (LOPD), and Others), and Region 2024-2032”, offers a comprehensive analysis of the industry, which comprises insights on the market. The global Pompe disease treatment market size reached US$ 1.0 Billion in 2023. Looking forward, IMARC Group expects the market to reach US$ 1.3 Billion by 2032, exhibiting a growth rate (CAGR) of 2.58% during 2024-2032.
Industry Trends and Drivers:
- Increasing awareness and early diagnosis:
The Pompe disease treatment market is primarily driven by the increasing awareness about this condition among healthcare professionals and the general public. Pompe disease, a rare genetic disorder caused by the deficiency of the enzyme acid alpha-glucosidase, can lead to severe muscle weakness and respiratory issues if left untreated. Increased education and outreach efforts by patient advocacy groups, medical associations, and healthcare providers are helping to improve recognition of the symptoms of the disease and facilitating earlier diagnosis. Early identification is essential as it allows for timely intervention, which can significantly improve patient outcomes. This rising awareness is leading to more patients being diagnosed and subsequently treated, thereby driving demand for effective treatment options.
- Advancements in treatment options:
The development and approval of innovative treatment therapies are significantly influencing the growth of the Pompe disease treatment market. Enzyme replacement therapy (ERT), particularly the use of alglucosidase alfa (Myozan), has become the standard treatment, helping to alleviate symptoms and improve quality of life for patients. Moreover, ongoing research and clinical trials are exploring additional therapies, such as gene therapy and small molecule drugs, which hold the potential to provide more effective and durable treatment solutions. As these advancements continue to emerge, they are enhancing the treatment landscape for Pompe disease, attracting investment from pharmaceutical companies, and fostering further innovation in the field.
- Growing support from regulatory agencies and health organizations:
Support from regulatory agencies and health organizations is vital in shaping the growth of the Pompe disease treatment market. Organizations such as the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have implemented various policies to expedite the approval of therapies for rare diseases, including Pompe disease. Moreover, orphan drug designations, which provide incentives for the development of treatments for rare conditions, encourage pharmaceutical companies to invest in research and development (R&D). Furthermore, public health initiatives aimed at improving access to rare disease treatments are enhancing the availability of Pompe disease therapies, ensuring that more patients can receive the care they need.
For an in-depth analysis, you can request a sample copy of the report: https://www.imarcgroup.com/pompe-disease-treatment-market/requestsample
Competitive Landscape:
The competitive landscape of the market has been studied in the report with detailed profiles of the key players operating in the market.
- Amicus Therapeutics Inc.
- Audentes Therapeutics Inc. (Astellas US Holding Inc.)
- Oxyrane UK Limited
- Sanofi S.A.
- Spark Therapeutics Inc
Pompe Disease Treatment Market Report Segmentation:
Breakup By Treatment:
- Enzyme Replacement Therapy (ERT)
- Substrate Reduction Therapy (SRT)
- Chaperone-Advanced Replacement Therapy (CART)
- Others
Enzyme replacement therapy (ERT) accounts for the majority of shares due to its established efficacy in addressing the underlying enzyme deficiency that characterizes the condition.
Breakup By Route of Administration:
- Oral
- Intravenous
- Others
Based on the route of administration, the market has been segregated into oral, intravenous, and others.
Breakup By Distribution Channel:
- Hospital and Clinics Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Hospital and clinics pharmacies hold the majority of shares due to their direct access to patients requiring specialized therapies, such as enzyme replacement therapy.
Breakup By Indication Type:
- Infantile-Onset Pompe Disease (IOPD)
- Classic Infantile Form
- Non-Classic Infantile Form
- Late-Onset Pompe Disease (LOPD)
- Others
Late-onset Pompe disease (LOPD) exhibits a clear dominance due to its higher prevalence compared to the infantile form, resulting in a larger patient population seeking treatment.
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America holds the leading position owing to a large market for Pompe disease treatment driven by its advanced healthcare infrastructure, leading to early diagnosis and access to specialized treatment options.
Ask Analyst for Customization: https://www.imarcgroup.com/request?type=report&id=6708&flag=C
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provides a comprehensive suite of market entry and expansion services. IMARC offerings include a thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape, and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Spellen
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


